Insect–plant interactions
The vast majority of living insect species belong to Holometabola and undergo complete metamorphosis; the vast majority of living plant species are angiosperms, which use flowers to reproduce and have greater hydraulic capacities than anything that came before them. These two massively successful groups did not diversify in synchrony: holometabolous insects originated over 300 million years ago and became dominant on the landscape long before the first fossil evidence of angiosperms, which rose to prominence only about 100 million years ago. Therefore, for over half of insect history, Holometabola predominated in insect populations but angiosperms were scarce or absent. This under-studied interval provides a natural experiment into whether the ecological associations we see today result from angiosperms’ unprecedented diversity, productivity, and specialized associations with insect herbivores and pollinators—or if, instead, insects would have had equally diverse associations with gymnosperms if angiosperms had never evolved.
No one knows who they were or what they were doing
But their legacy remains
Hewn into the living rock– Nigel Tufnel, 1982
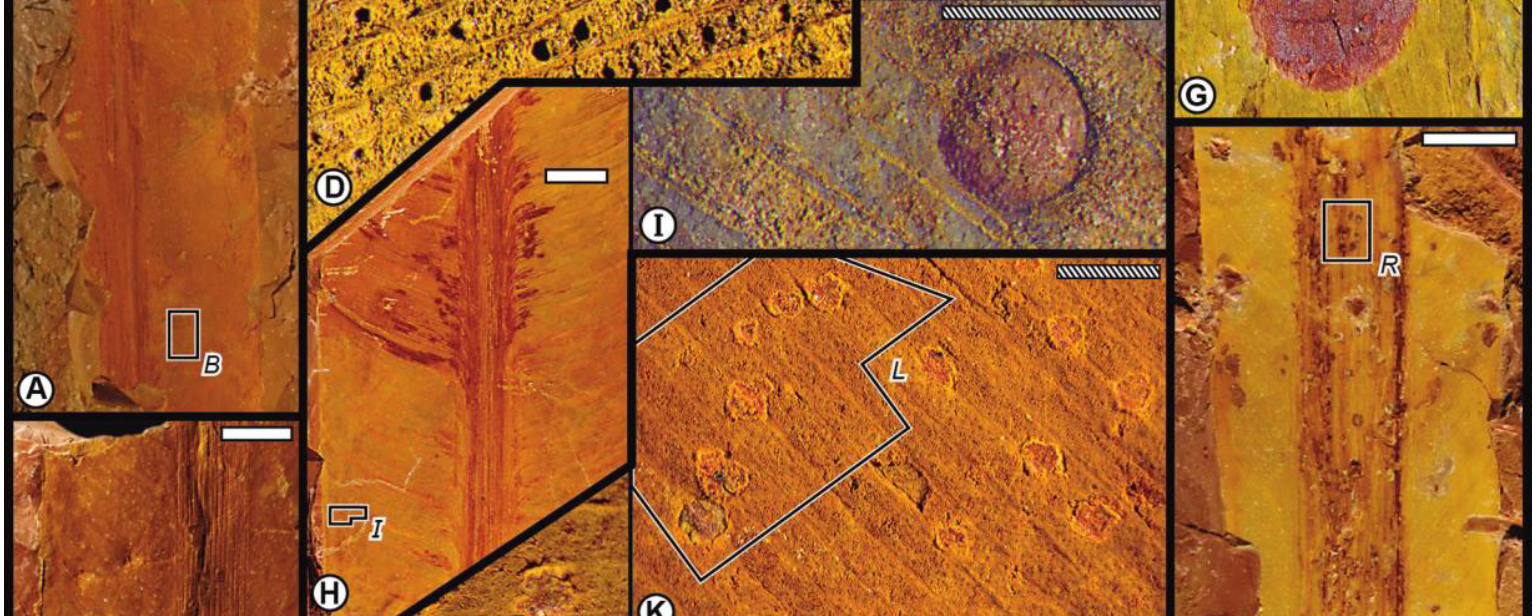
By examining Paleozoic plant fossils, I have found that specialized forms of insect herbivory (galls), which overwhelmingly occur on angiosperms in modern ecosystems, originated barely 25 million years after winged insects appear in the fossil record. I found that the insects that engaged in this behavior belong to the most ancient insect group that induces galls in modern ecosystems. In other words, the origin of this feeding behavior was constrained by insect evolution rather than plant evolution.
I also have a longstanding interest in developing new quantitative methods for the study of insect–plant interactions in the fossil record, inspired largely by the work of marine paleontologists. I first devised a robust framework for quantifying changes in insect herbivory throughout geologic time and then developed new methods for quantifying the uncertainty that results from uneven sampling effort among localities. Because the statistical trends in insect herbivory gleaned from paleontological studies are partly a function of which leaves become fossilized, I collaborate with paleobotanists to better understand the preservation of plants in the fossil record.
I applied my quantitative background to the role of angiosperms in insect evolution by reconstructing the taxonomic diversity of insects in deep time using a method that has been primarily used in the study of marine invertebrates. I found that although insects diversified markedly at the family level during the Cretaceous Period—when angiosperms went from rare to ubiquitous—their higher-level diversification predates the radiation of angiosperms by tens of millions of years. Although the hypothesized net speciation of insects during the past 60–100 million years has been attributed to herbivores’ and pollinators’ interactions with angiosperms, the net diversification of insect families occurred far too early in the geological record to have been driven by angiosperms and is best attributed to an evolutionary arms race between parasitoid wasps and the other insects that they target.
The origin of insect wings in geologic context
My research addresses the questions of when winged insects originated and why they appear to have evolved many tens of millions of years later than other major groups of land-dwelling arthropods (centipedes, millipedes, scorpions, etc.) and after the origin of tall trees whose canopies can be reached far more easily by flying than walking. To understand the near absence of insects in the fossil record more than 325 million years ago, I combined geochemical modeling with analyses of rock volume and of the insect body parts preserved in the fossil record. I found, contrary to previous estimates, that atmospheric oxygen was not particularly low during any point when winged insects were hypothesized to exist but have not been found in the fossil record—casting doubt on the hypothesis that pO2 constitutes one of the major constraints on insect evolution.
Furthermore, I conducted the first quantitative analysis of the types of sedimentary rocks preserved through time to evaluate the contention that insects are absent from this long stretch of the fossil record because of preservational biases rather than real biological phenomena. I found that insects are nearly absent from the fossil record more than 325 million years ago simply because they had not yet evolved wings. This finding shows that winged insects are one of the few higher clades whose age can be constrained with a “hard maximum,” or oldest possible age. The ability to place a hard maximum on winged insects’ age permits analyses of the extent to which rates of evolution in this group have changed throughout deep time, a question that I am currently addressing in collaboration with systematists.